The surface energy of a solid is a measure of how easily a surface can be wetted by a liquid and gives an indication of the expected adhesion properties on that solid. The surface energy can be determined experimentally by carrying out contact angle measurements with several test liquids.
What is the surface energy of a solid?
The surface energy describes a special case of interfacial energy, namely at the surface of a solid to a gas (usually ambient air). The surface energy is a measure of how easily the solid surface can be wetted by a liquid. In addition, the surface energy influences how well solid or liquid materials adhere to the solid surface.
Important application examples for surface energy measurements
In practice, a higher surface energy of solids indicates better wetting and thus better adhesion. If a solid surface with high surface energy comes into contact with a water drop, a low contact angle forms. This means that the water drops spread on the surface. Glass, ceramics and many metals are examples of solids whose surfaces have a high surface energy naturally.
A low surface energy indicates poor wetting and thus poorer adhesion. When in contact with a water drop, a high contact angle is formed. This means that the drop of water rests on the surface and does not spread. Many plastics [Application Note: Polymer Substrates] originally have a low surface energy. Materials with a low surface energy require pre-treatment, before they can be further processed - for example, printed or glued. Other materials are specially developed to have water-repellent properties - examples are water-repellent textile coatings or window glass.
How can the surface energy of a solid be measured?
The surface energy of a solid can be determined by measuring several contact angles with a contact angle meter and the sessile drop method. Specifically, one measures the contact angles of at least two test liquids whose surface tension is known. Popular test liquids are water, diiodomethane, ethylene glycol, or thiodiglycol.
The surface energy of a solid can be calculated using these contact angle measurements based on various models. Such models include calculations of the different interactions that take place between the solid and the liquid. Especially well-established is the division into dispersion and polar interactions.
The most frequently used model comes from Owens, Wendt, Rabel and Kaelble. It is also called the OWRK model. It contains the geometric means of the dispersion and polar components of the surface tension of the liquid and the surface energy of the solid:
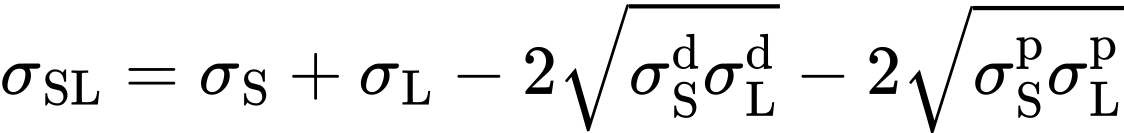
The next step is to refer to Young's equation (Contact angle explained), which expresses the relationship between contact angle, surface tension, and surface energy:
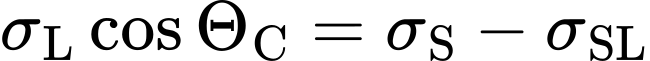
If the OWRK model is now substituted into Young's equation, this can be converted into a linear equation in the form y = mx+c:
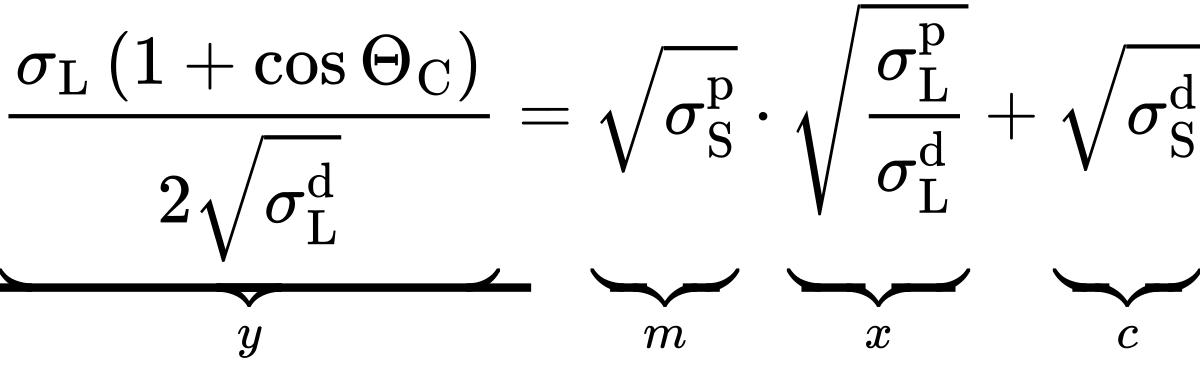
The following symbols have been used:
- σSL: Interfacial tension between liquid and solid
- σS: Surface energy of the solid
- σSd: Dispersion component of the surface energy of the solid
- σSp: Polar component of the surface energy of the solid
- σL: Surface tension of the test liquid
- σLd: Dispersion component of the surface tension of the test liquid
- σLp: Polar component of the surface tension of the test liquid
- θC: equilibrium contact angle