Model experiment: interfacial energy and interfacial tension are equivalent for liquids
At the interfaces between a liquid and a gas or between two liquids, the two concepts - interfacial energy and interfacial tension - can be equated. This can be ascertained with the help of a model experiment.
For this purpose, a thin film of liquid, for example soap suds, is stretched in a U-shaped wire, to the open side of which a movable stirrup is attached (see Figure 2). An equilibrium is established between the surface forces of the liquid film and the weight of the stirrup. In order to move the stirrup by Δs and thus increase the surface area by ΔA = 2bΔs (double the rectangular area, since the film has a front and a back), the force F must be applied.
Through the liquid surface, a tensile stress acts on the yoke, which counteracts the surface enlargement. This tensile stress is also called surface tension. Against this tensile stress, the work ΔWStir is performed on the stirrup to increase the surface area:
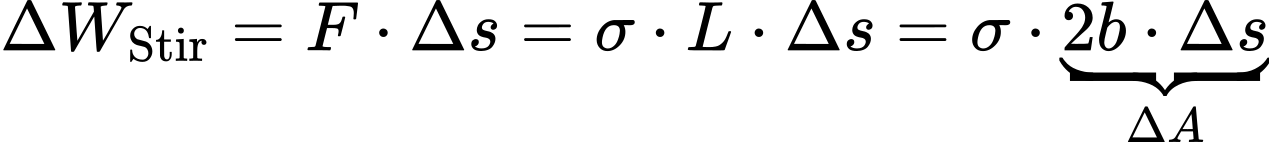
For liquids, the work ΔWStir performed on the stirrup is equal to the work ΔWSur needed to increase the surface area:
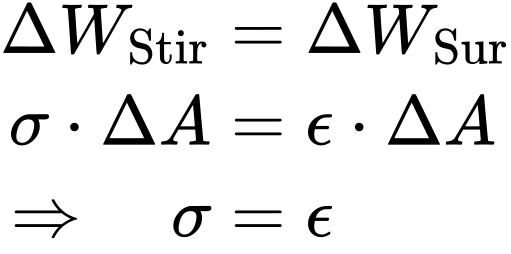
This means that for liquid-gaseous interfaces, the surface tension σ and the surface energy ε are equal. The same considerations apply to a liquid-liquid interface.
Why does the equivalence of interfacial tension and interfacial energy not apply to solids?
The equivalence between interfacial energy and interfacial tension only applies to liquids, because in a liquid, the atoms or molecules can be displaced within the phase without additional work. If one were to carry out the model experiment with a solid, one would also have to take into account the work required to displace the atoms or molecules within the phase. Therefore, the equation of surface energy and surface tension is not possible for solids.